New Laboratory Equipment, Fermentation
GMI’s GMP-Compliant GPC Bio Fermentation Solutions
Introduction
In bioprocessing, large-scale fermentation serves as a cornerstone for producing a wide array of biopharmaceuticals, industrial enzymes, and bio-based products. As the demand for biologics continues to soar, ensuring compliance with Good Manufacturing Practice (GMP) standards is paramount to safeguarding product quality, integrity, and patient safety. At GMI, we recognize the critical role that GMP compliance plays in large-scale fermentation operations. This blog post highlights how our GPC Bio Fermentation solutions adhere to GMP guidelines, empowering bioprocessors to achieve excellence in biomanufacturing.
Understanding GMP in Large-Scale Fermentation
GMP regulations establish stringent guidelines for manufacturing pharmaceuticals and biologics, encompassing various aspects such as facility design, equipment qualification, process validation, and quality control. Compliance with GMP ensures that biopharmaceutical products are consistently produced and controlled according to established quality standards, minimizing the risk of contamination, cross-contamination, and deviations that could compromise product safety and efficacy.
gpc bio from gmiGMI’s Commitment to GMP Compliance
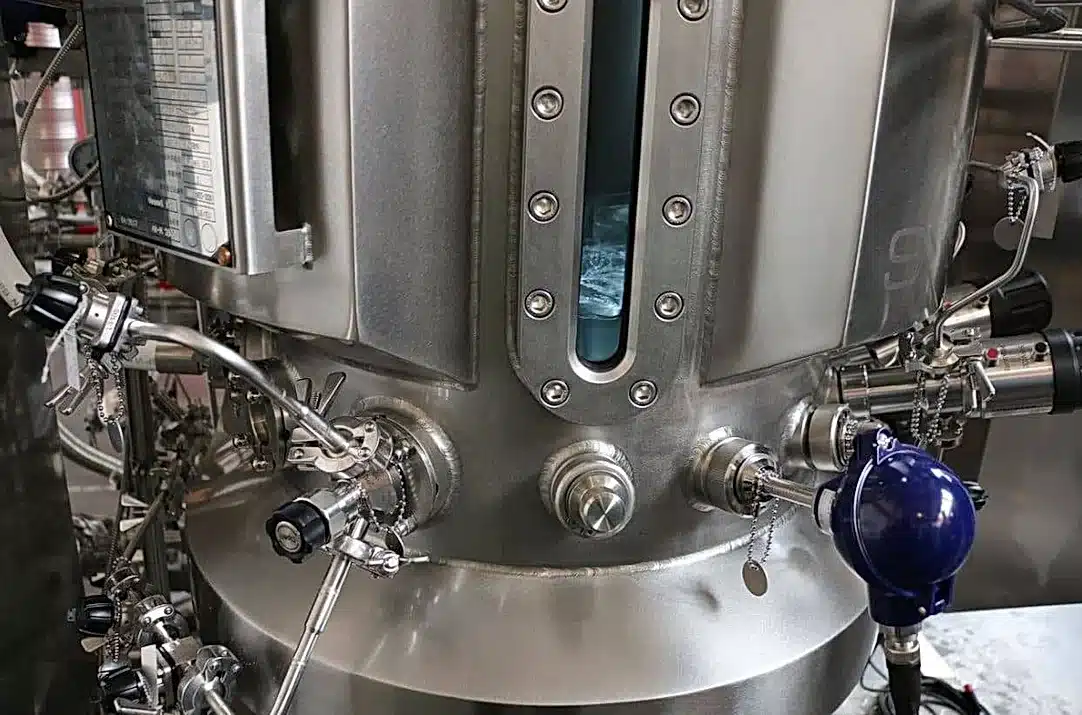
At GMI, we are committed to providing bioprocessors with cutting-edge fermentation solutions that meet the highest standards of GMP compliance. Our GPC Bio Fermentation systems are designed and engineered with GMP principles in mind, offering a robust platform for the scalable production of biologics and bio-based products. From selecting materials and components to designing the fermentation vessels and control systems, every aspect of our GPC Bio Fermentation solutions is meticulously crafted to ensure compliance with GMP requirements.
Key Features of GPC Bio Fermentation
Our GPC Bio Fermentation systems boast a host of features that align with GMP guidelines, including:
- Material Selection: Utilization of high-quality, GMP-compliant materials for construction, ensuring durability, cleanliness, and compatibility with bioprocess applications.
- Sterilization: Robust sterilization protocols and techniques to eliminate microbial contaminants and maintain aseptic conditions throughout fermentation.
- Process Control: Advanced automation and monitoring capabilities for precise control over crucial fermentation parameters such as temperature, pH, agitation, and dissolved oxygen levels.
- Documentation and Traceability: Comprehensive documentation practices and data logging functionalities to facilitate process traceability, batch recordkeeping, and regulatory compliance.
- Validation and Qualification: Thorough validation and qualification protocols to demonstrate the performance, reliability, and reproducibility of our GPC Bio Fermentation systems.
Advantages of GMI’s GMP-Compliant Solutions
By investing in GMI’s GPC Bio Fermentation solutions, bioprocessors can benefit from:
- Assurance of product quality, integrity, and compliance with regulatory requirements.
- Enhanced operational efficiency, scalability, and reproducibility of fermentation processes.
- Mitigation of risks associated with contamination, deviations, and non-compliance.
- Confidence in the reliability, performance, and long-term sustainability of fermentation operations.
Conclusion
At GMI, we understand the critical importance of GMP compliance in large-scale fermentation and biomanufacturing. Our GPC Bio Fermentation solutions represent the pinnacle of GMP-compliant engineering, offering bioprocessors a reliable and scalable platform for producing biopharmaceuticals and bio-based products. With our unwavering commitment to quality, innovation, and customer satisfaction, GMI is proud to support the biopharmaceutical industry in its mission to advance healthcare and biotechnology through excellence in biomanufacturing.
for more information, click here